How to Draw a Plane of Symmetry for Compounds Orgo
Question: Are these molecules enantiomers, diastereomers, or the aforementioned?
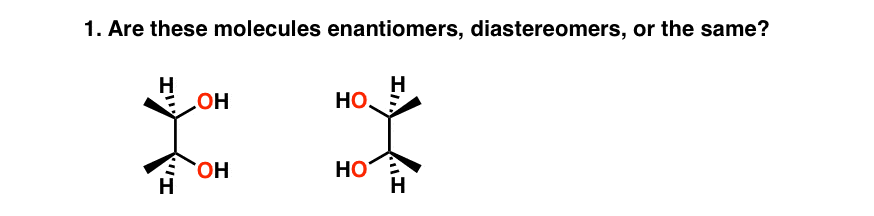
If you immediately recognized this as a molecule with an internal plane of symmetry (and thus an achiral molecule, incapable of having an enantiomer), congratulations. If not, yous just fell into The Meso Trap.
The Meso Trap is a common feature on exams and tests to make sure that you empathize the concept of chirality and that you are paying attending.
Just to make things clear, a meso chemical compound is a molecule that has chiral centers only too has an internal airplane of symmetry. This renders the molecule achiral: it does non have an enantiomer, and it does non rotate plane polarized light .
Information technology is similar to a phenomenon establish in certain two-headed, two-tailed cats.
The Meso Trap usually comes upwards in conjunction with the testing of another important skills, notably:
1) identifying types of isomers
2) manipulating various types of chemical diagrams
3) determining (R)/(S) nomenclature for given stereocenters
four) identifying the stereochemistry of various types of reactions
These are all key skills. We are non talking near lilliputian stuff here.
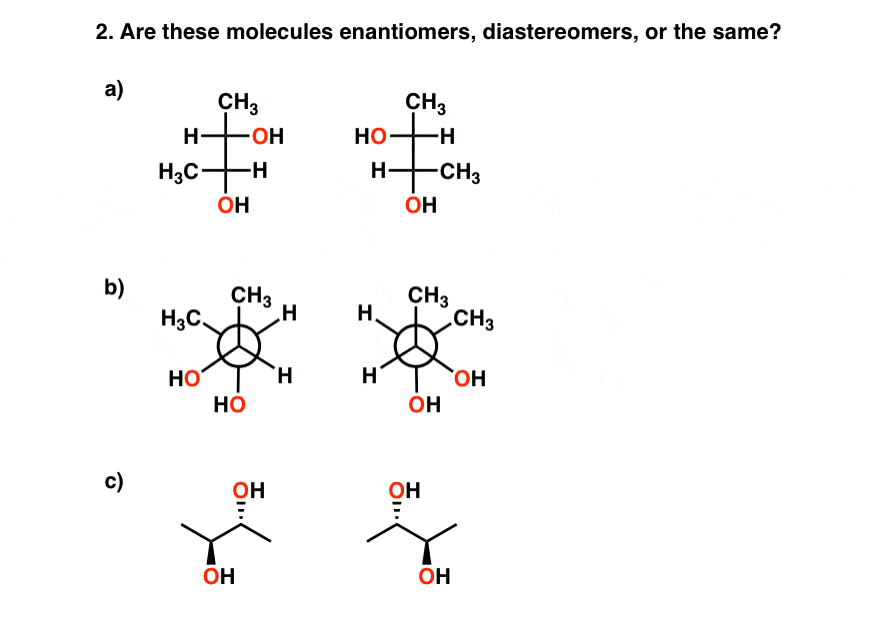
It tin come upward in glaringly obvious ways (like in the above example) or in a number of other ways, equally in these examples, where the meso compound is not nearly as obvious, and drawn in different projections.
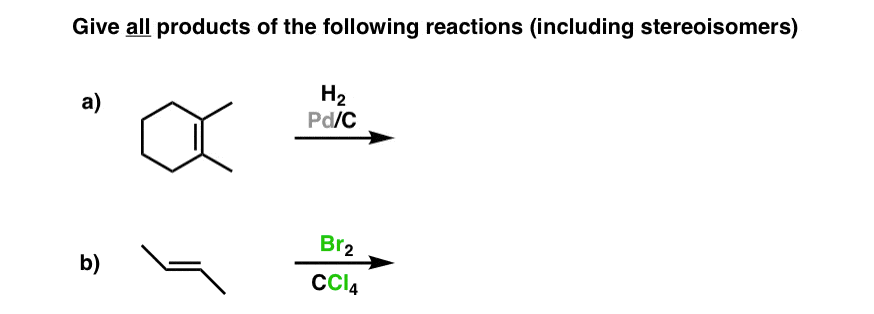
The Meso Trap can also sneak upwards on you lot in other means, like when you're being asked for the products of a reaction. In the following instance, you might exist tempted to draw in not only the product of the reaction just also its enantiomer. In this case, however, the product is a meso compound… significant that there is only one product.
How To Avoid Falling into The Meso Trap
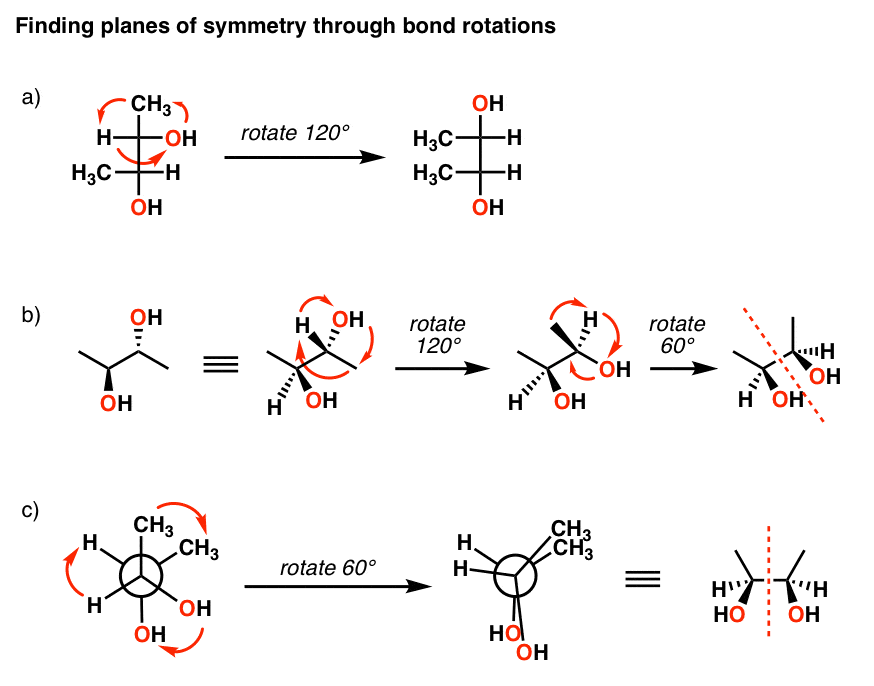
Identifying a meso compound means being able to place a plane of symmetry in a molecule. It'due south important to realize that this tin cut either through a bail or through an atom, as in the following examples.
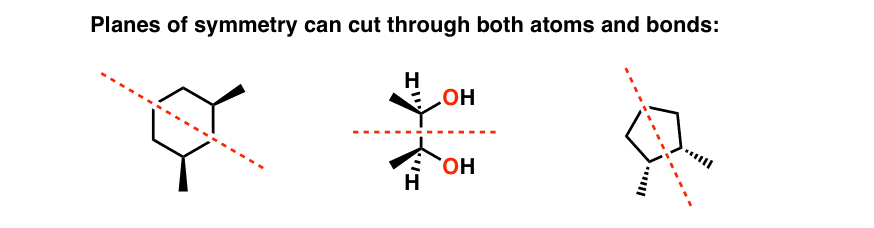
What if the airplane of symmetry isn't obvious? That'south when you really take to demonstrate that you lot accept a few important skills – similar the ability to recognize molecules fatigued in dissimilar projections, how to practise bond rotations, and how to recognize (R)/(S) designations.
There are really two basic strategies for identifying a meso compound if it isn't drawn in an obvious configuration.
The path of greatest resistance, but greatest advantage is to learn to master how to perform bond rotations on line-wedge diagrams, Newman projections, Fischer projections, besides equally to be able to convert between the three types of drawings as necessary. In the beginning stages, using a model kit to check your work is invaluable. With these skills you can take any structure given to you lot and be able to rotate the bonds in a way to examination whether the two molecules are mirror images.
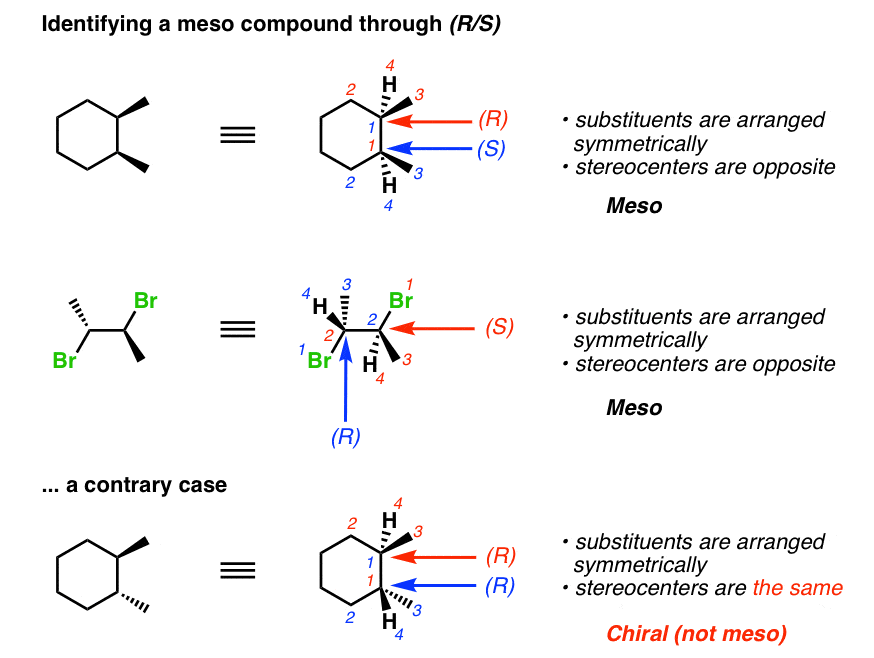
A slightly easier method to determine whether a given compound is meso is to take advantage of a simple principle: meso compounds have an internal mirror airplane. That is to say that non only must 1) every carbon on each side of a mirror plane accept the same substituents, simply in addition 2) every (R) stereocenter on i side of the mirror airplane must be balanced by an (S) stereocenter on the contrary side. So if you can chop-chop make up one's mind (R)/(S), y'all can besides quickly decide whether a given compound is meso without having to practise bail rotations. If the stereocenters aren't opposite, it can't exist meso. Depending on the complexity of the molecule, this tin be a much faster way to do things.
The fundamental ingredient in avoiding falling into the Meso Trap is paranoia. When asked if molecules are diastereomers/enantiomers/the same, ask yourself – "is this a symmetric molecule?". Every time. After doing an addition reaction to a double bond, inquire yourself: "is this a symmetric molecule?". This comes upward a lot more frequently than you'd expect – peculiarly on tests.
It also comes up later in NMR, where recognizing a plane of symmetry tells you how many distinct proton or carbon signals you lot will encounter.\
Answers:1) the same (meso) 2) a), b), c) are all examples of the same (meso) compounds, drawn differently. iii) a gives cis-dimethylcyclohexane (a meso chemical compound), b) gives (R,S)-two-3-dibromobutane, besides a meso compound.
Source: https://www.masterorganicchemistry.com/2011/01/12/the-meso-trap/
0 Response to "How to Draw a Plane of Symmetry for Compounds Orgo"
Post a Comment